Comprehensive List of Researchers "Information Knowledge"
Department of Complex Systems Science
- Name
- NAGAOKA, Masataka
- Group
- Material Informatics Group
- Title
- Professor
- Degree
- Dr. of Engineering
- Research Field
- Theoretical chemistry / Computational science / Nonequilibrium phenomenology

Current Research
"Computing" Material Transformation ! - Theoretically and Visually -
OUTLINEWe have been studying the frontiers of computational science in chemical reaction dynamics informatically and visually. While the word chemistry might bring to mind images of a laboratory, white robes, and flasks, one major field of today's computational science is occupied with such f"chemistry," which seems at a glance to be quite irrelevant to computers. In fact, computational chemistry and theoretical chemistry, promoted by the heavy use of computer technology, are traditional in the world-class research conducted in Japan. This can be partially recognized in the 1981 Nobel Prize for Chemistry awarded to Fukui for the "Frontier Electron Theory."
TOPICS
(1) Two Approaches of Chemical Reactions There are two main approaches in computational science of chemical reactions. One is the "chemical kinetics" approach. It determines the reaction mechanism of the product experimentally obtained and designs the reaction mechanism to obtain the product that is to be newly synthesized. This is done by establishing, according to the expected reaction mechanism, simultaneous differential equations concerning several kinds of densities of a molecular species (e.g., molecular number density or partial pressure, etc.) and then solving them. It can be said that this is an approach which is experimentally oriented toward reproducing an experimental value such as the yield. The other approach is "quantum chemistry," which treats the molecule from the microscopic viewpoint and deals with the cleavage and the formation of chemical bonds that take place during the reaction at the atomic level. From this standpoint, the purpose is to understand the molecular structure of the product and the rearrangement mechanism of such atoms under the reaction, done by determining a molecular chemical reaction at the atomic level and connecting it to a new molecular synthetic design. Essentially, although a chemical phenomenon that happens in a test tube is unique, there are now two approaches according to the difference in standpoint.
(2) Molecular Frictional Theory In our laboratory we have been working mainly with the latter, and have been promoting computational scientific research on material chemical reaction dynamics in condensed systems (i.e., such molecular aggregates as liquids or solids) on the basis of the techniques of quantum and theoretical chemistry. In 1991 we advocated the chemical reaction molecular dynamics method, which was not yet known worldwide, and developed the technique of incorporating the microsolvent effect and nonequilibrium nature from first principles. Moreover, we developed the molecular frictional theory using a molecular hamiltonian, which was established on the basis of the electronic state theoretical information, enabling us to consider the origin of a frictional phenomenon in molecular terms, i.e., intramolecular or intermolecular interactions.
(3) Free Energy Gradient Method Furthermore, we developed the free energy gradient method as a structure optimization method for a solvated or adsorbed molecule, and succeeded for the first time in the full optimization of the transition state structure of a Menshutkin reaction in solution, paving the way for wide use.
FUTURE WORK
Recently, since a number of ultra-fast chemical phenomena on the sub-pico to femtosecond scale have come to be quantitatively observed experimentally, we aim, at present, to construct a computational scientific methodology that enables us to understand such super-multi-degrees-of-freedom material systems at an atomic resolution, which can be observed experimentally, and that lead to the microscopic elucidation of (i) nonequilibrium phenomena and (ii) an understanding life phenomena. Our long-term goal is to smoothly connect the first approach with the second one.
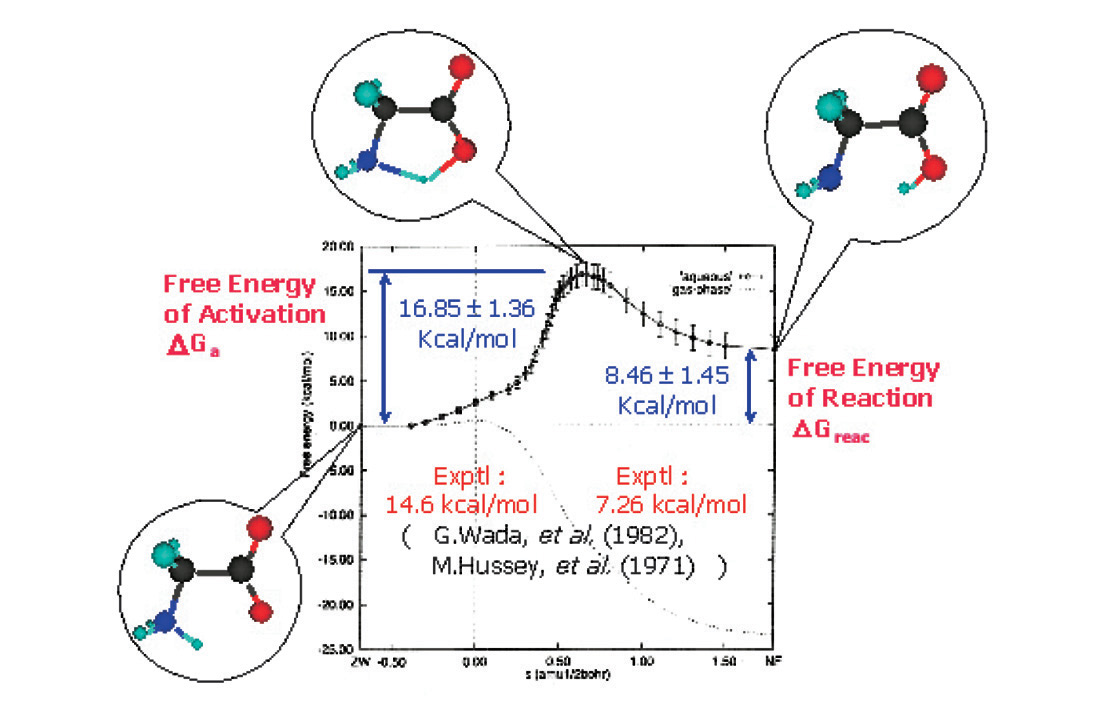
Figure : Free energy diagram of the isomerization reaction of glycine molecule in aqueous solution
Career
- 1988 Dr. Eng., Graduate School of Engineering, Kyoto University. 1988 Research Associate, Institute for Fundamental Chemistry. 1998 Associate Prof., Graduate School of Human Informatics, Nagoya University. 2002 Prof.
- 2003 Prof., Graduate School of Information Science, Nagoya University.
Academic Societies
- CSJ
- PSJ
- MSSJ
- BSJ
- ACS
- APS
Publications
- Coarse-Grained Approach to Nonequilibrium Molecular Dynamics : Application to Relaxation process of Vibrationally Excited Hydrogen Fluoride in Aqueous Solution, Chemical Physics Letters, 407, 444 (2005).
- Structure Optimization via Free Energy Grandient Method : Application to Glycine Zwitterion in Aqueous Solution, Journal of Chemical Physics, 113, 3519 (2000).
- Origin of the Transition State on the Free Energy Surface : Intramolecular Proton Transfer Reaction of Glycine in Aqueous Solution, Journal of Physical Chemistry A, 102, 8202 (1998).








