Comprehensive List of Researchers "Information Knowledge"
Department of Complex Systems Science
- Name
- KOGA, Nobuaki
- Group
- Material Informatics Group
- Title
- Professor
- Degree
- Dr. of Engineering
- Research Field
- Computational quantum chemistry

Current Research
Theoretical Studies of Electronic Structures of Molecules and Chemical Reactions
OUTLINETo date we have theoretically studied the structures of molecules and the profiles of chemical reactions using quantum chemical computational methods such as the ab initio (non-empirical) molecular orbital method and the density functional theory method. These first-principles calculations would give information on the electronic as well as geometric structures, which are expected to be useful in designing molecules and chemical reactions. With the theoretical calculations we could clarify the nature of transient species and transition states, which are difficult to study experimentally, and recognize the factors controlling chemical reactions : the driving force promoting a reaction, what realizes selectivity, and so on. We are investigating with quantum computational methods mechanisms of elementary organometallic reactions and a whole catalytic cycle constructed by combining elementary reactions. We are also interested in analyzing properties of molecules
TOPICS
(1) Organometallic Reactions
Specially, we focus on the chemical reactions in transition metal complexes, which are, in some cases, the elementary reactions observed in catalytic reactions mediated by transition metal complexes. By combining elementary chemical reactions, we can theoretically construct an entire catalytic cycle. Figure 1 shows the results obtained in one recent study in this field. It was experimentally found that the strong CC bond in CH3CN can be broken by an iron complex with a silyl ligand, CpFe(CO)2(SiMe3) (Cp=C5H5, Me=CH3). The theoretical calculations clarified the most plausible mechanism, in which the insertion of the CN bond of side-on CH3CN into the Fe-Si bond with a small activation energy of 4.0 kcal/mol and the successive C-C bond cleavage of CH3CN on the Fe coordination sphere with an activation energy of 15.0 kcal/mol, take place to give Cp(CO)MeFe(CNSiMe3). Both steps are simple because the latter step is isoelectronic to decarbonylation, and in the former the N-Si bond is formed between oppositely charged N and Si atoms. In fact, when the silyl group is replaced by a methyl group, the activation energy was calculated to be much higher, indicating that the silyl group assists the CC bond activation. The role of the silyl ligand was clearly demonstrated. Transition metal atoms can have several oxidation states and bear various ligands, so there are many possible geometrical and electronic structures. Accordingly, quantum chemical calculations are time-consuming and not easy. However, we could perform theoretical studies by properly modeling a reaction system.
(2) Analysis of electronic structure of molecules
We analyzed the electronic structure of various molecules by designing new analytical tools : i) We developed a molecular electrostatic potential bond critical point model for atomic and group electronegativities to show that the new scale of electronegativities agrees well with, and is even more accurate than, conventional electronegativity scales. ii) Also, we have proposed values for the molecular electrostatic potential minimum of the lone pair region of substituted phosphine ligands as a quantitative measure of the electronic effect of phosphine ligands. iii) We devised a new thermodynamic scale of aromaticity for various organic molecules using isodesmic reactions, which offers an easy way to compare the aromaticity of molecules in different categories. iv) Since the molecular orbitals generally delocalize over the whole molecule, analysis may be difficult, especially in the case of large molecules. Therefore, we are developing a method to analyze complicated electronic structures in terms of intramolecular interaction.
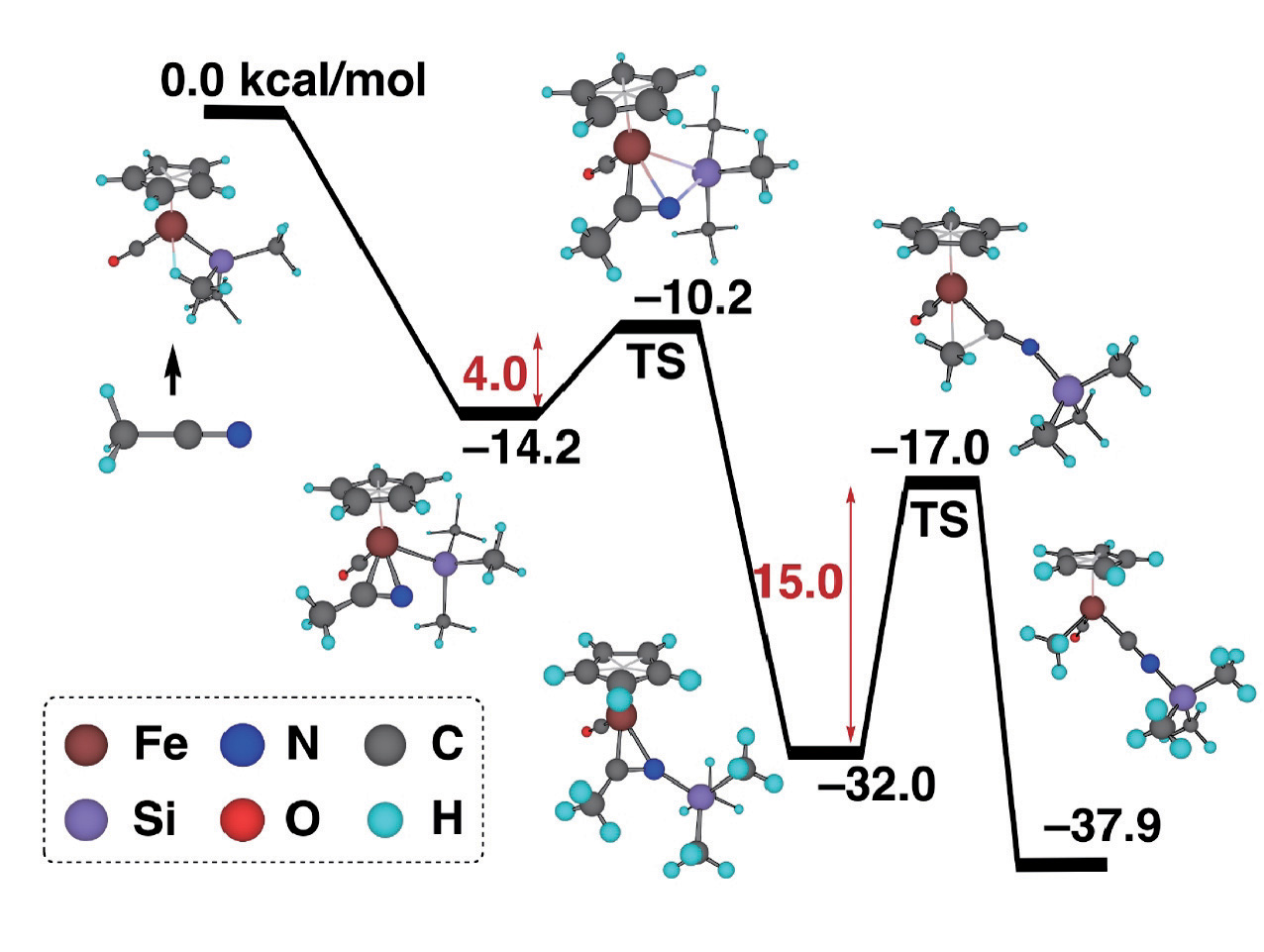
Figure : Energy profile of an organometallic reaction
Career
- Nobuaki Koga received his Dr. of Engineering degree from Kyoto University in 1987.
- He was a Research Assoc. at the Institute for Molecular Science from 1986 to 1993 and an Assoc. Prof. at Nagoya University from 1993 to 1998.
- Since 1998, he has been a Prof. at Nagoya University.
Academic Societies
- Chemical Society of Japan
- American Chemical Society
Publications
- C. H. Suresh and N. Koga, Orbital Interactions in the Ruthenium Olefin Metathesis Catalysts, Organometallics, 23, 76-80 (2004).
- C. H. Suresh and N. Koga, Aromaticity-Driven Rupture of CN Triple and CC Double Bonds : Mechanism of the Reaction Between Cp2Ti(C4H4) and RCN, Organometallics, 25, 1924-1931 (2006).
- A. A. Dahy and N. Koga, Theoretical Study on the Reaction Mechanism for the Formation of 2-Methylpyridine Co(I) Complex from Cobaltacyclopentadiene and Acetonitrile, Organometallics, 28, 3636 (2009).








